Process Development
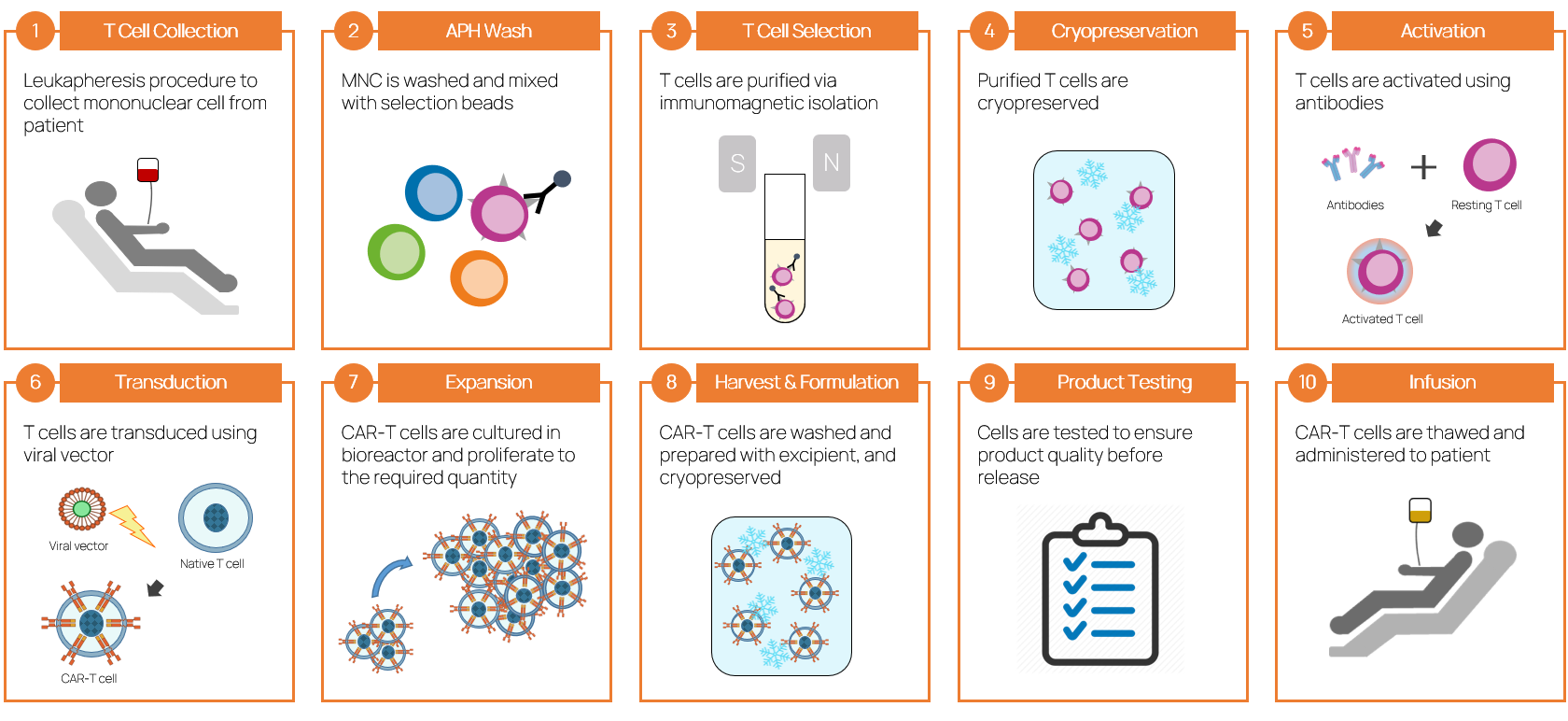
Manufacturing Process of CAR-T Therapies
- “The process is the product” - the manufacturing process of CAR-T therapies significantly influences product characteristics, and our processes are critical to production success
|
|
CAR-T |
Biologics |
|
Product |
Living cells as product |
Generated by cultured cells |
|
Product Definition |
Product definition continues to be refined throughout clinical studies and CMC development |
Well-defined or definable |
|
Cells Variability, Heterogeneity |
Large variety of cells |
Monoclonal cell lines with small variance |
|
Source of Starting Material |
Complicated process and logistics in extracting blood from patients to infusion |
Cryopreserved MCB/WCB |
|
Process |
- Product-specific sequence of procedures and operations - Little/no hold times between operations - Little/no product purification |
- Well-defined unit operations with product-specific operating parameters - Validated hold times between unit operations common - Extensive product purification operations |
|
Process Scale |
Scale out to meet demands |
Scale up to meet demands |
Zhangjiang R&D Center in Shanghai

- Approximately 2,500 square meters
- Has established an integrated cell therapy product development platform, including cell therapy process development, lentiviral vector process development, plasmids process development, sterility lab, flow cytometry assays, in vitro cell functionality assays, and molecular assays
- Recognized as a “Foreign-invested R&D center” by Shanghai Municipal People’s Government in 2019
